is methylene chloride polar Methylene chloride: properties, structure & uses
In this post, we will explore the concept of polarity in molecules and specifically focus on a well-known chemical compound called methylene chloride. Methylene chloride, also known as dichloromethane, is a colorless liquid with a sweet smell. It is commonly used as a solvent in various industrial and manufacturing processes.
Is Methylene Chloride Polar or Nonpolar?
Before we delve into the polarity of methylene chloride, let’s first understand what polarity means in the context of molecules. Polarity refers to the distribution of electrical charge within a molecule. A molecule is said to be polar if it has a positive and negative end, resulting from an uneven distribution of electrons.
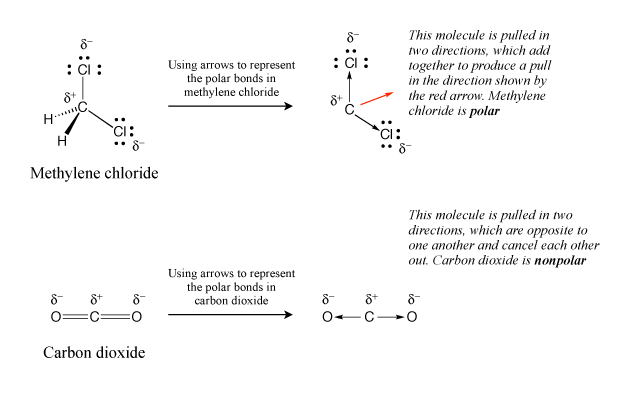
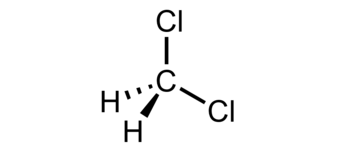
So, is methylene chloride polar or nonpolar? To answer this question, we can take a closer look at its molecular structure. Methylene chloride has a linear structure with a carbon atom at its center. This carbon atom is bonded to two chlorine atoms and two hydrogen atoms.
 Now, let’s analyze the electronegativity of the atoms involved. Electronegativity is a measure of an atom’s ability to attract electrons towards itself. Chlorine is more electronegative than carbon and hydrogen, which means it has a stronger pull on the shared electrons in the chemical bond.
Now, let’s analyze the electronegativity of the atoms involved. Electronegativity is a measure of an atom’s ability to attract electrons towards itself. Chlorine is more electronegative than carbon and hydrogen, which means it has a stronger pull on the shared electrons in the chemical bond.
Due to this difference in electronegativity, the electrons in the carbon-chlorine bonds are pulled closer to the chlorine atoms, resulting in a partial negative charge on the chlorine atoms and a partial positive charge on the carbon atom. As a result, methylene chloride is a polar molecule.
Molecular Polarity
Methylene chloride is just one example of a polar molecule, and it is important to understand the implications of molecular polarity. Polar molecules have different physical and chemical properties compared to nonpolar molecules.
One important property is their ability to dissolve in polar solvents. Since like dissolves like, polar solvents like water are effective in dissolving polar solutes like methylene chloride. This property makes methylene chloride a versatile solvent for many applications.
 Moreover, the polarity of a molecule also influences its boiling point and melting point. In the case of methylene chloride, its boiling point is relatively low (-97.4 °C or -143.3 °F), which makes it volatile and easily evaporated.
Moreover, the polarity of a molecule also influences its boiling point and melting point. In the case of methylene chloride, its boiling point is relatively low (-97.4 °C or -143.3 °F), which makes it volatile and easily evaporated.
It is important to note that polarity is not an all-or-nothing concept. There are also slightly polar molecules, where the difference in electronegativity is not as pronounced as in methylene chloride. This leads to a weaker polarity and a lesser ability to dissolve in polar solvents.
In conclusion, methylene chloride is a polar molecule due to the uneven distribution of electrical charge caused by the difference in electronegativity between the carbon and chlorine atoms. Understanding the polarity of molecules is crucial in various fields of chemistry, as it determines their behavior and interactions with other substances.
So next time you come across methylene chloride, you’ll know that it is indeed a polar compound!
If you are looking for Case Study: Removing caffeine from Coffee - Chemistry LibreTexts you’ve visit to the right web. We have 5 Pics about Case Study: Removing caffeine from Coffee - Chemistry LibreTexts like OCWR - Methylene Chloride: Read the Label and Don’t Trust Your Sense of, Methylene Chloride: Properties, Structure & Uses - Chemistry Uncovered and also Is methylene chloride polar or nonpolar. Read more:
Case Study: Removing Caffeine From Coffee - Chemistry LibreTexts
chem.libretexts.orgchloride methylene caffeine coffee study case processing removing polar libretexts molecule
Unit 1 - Elaboration - Molecular Polarity
 www.chem.uwec.edupolarity polar bonds molecule nonpolar example molecular carbon examples covalent dioxide figure
www.chem.uwec.edupolarity polar bonds molecule nonpolar example molecular carbon examples covalent dioxide figure
OCWR - Methylene Chloride: Read The Label And Don’t Trust Your Sense Of
 www.ocwr.govIs Methylene Chloride Polar Or Nonpolar
www.ocwr.govIs Methylene Chloride Polar Or Nonpolar
 www.bengislife.comchloride methylene polar nonpolar non polarity
www.bengislife.comchloride methylene polar nonpolar non polarity
Methylene Chloride: Properties, Structure & Uses - Chemistry Uncovered
 blog.carbanio.comchloride methylene dcm uncovered
blog.carbanio.comchloride methylene dcm uncovered
Chloride methylene caffeine coffee study case processing removing polar libretexts molecule. Is methylene chloride polar or nonpolar. Case study: removing caffeine from coffee